Motor Neuron Disease
See full list of projects
Neurodegeneration - Stimulating autophagy to improve intracellular proteostasis in MND - also offered as MBiomedSc
Supervisors: Dr Bradley Turner, Dr Bec Sheean
Project Site: Florey Institute, Kenneth Myer Building
Contact: Bradley Turner E: Bradley.turner@florey.edu.au T: 9035 6521
Project description: Motor neuron disease (MND) is a neurodegenerative and protein misfolding disorder linked to defects in proteostasis pathways, or protein homeostasis, within affected motor neurons. MND is associated with cytoplasmic accumulation and aggregation of key proteins (SOD1, TDP-43 and FUS) which are implicated in motor neuron death. Strategies that improve proteostasis and clear these misfolded proteins in motor neurons are therefore an attractive candidate therapeutic approach for MND. Our group is interested in autophagy, the main catabolic pathway in neurons that eliminates misfolded proteins, aggregates and damaged organelles by targeting these substrates to lysosomes for digestion.
This project will investigate the therapeutic effect and action of stimulating autophagy in genetic cell culture and mouse models of MND. The effects of newly identified autophagy enhancing drugs will be evaluated on clinical progression, neuropathology and misfolded and aggregated protein load in mouse models of MND. This project will employ transgenic mice, behavioural studies, advanced microscopy, immunohistological and biochemical techniques.
Neurodegeneration - Development of survival motor neuron gene therapy for spinal muscular atrophy
Supervisors: Dr Rebecca Sheean, Dr Bradley Turner
Project Site: Florey Institute, Kenneth Myer Building
Contact: Rebecca Sheean E: rebecca.sheean@florey.edu.au T: 9035 6567
Project description: Spinal muscular atrophy (SMA) is a progressive neuromuscular disorder and the leading genetic cause of infant death. SMA results from inactivation of the survival motor neuron 1 (SMN1) gene and retention of the SMN2 gene, leading to ubiquitous SMN protein deficiency and selective spinal motor neuron loss and muscle weakness. SMN is an essential factor for motor neurons regulating gene splicing and axonal functions important for motor neuron development. SMN gene replacement or upregulation using viral vectors or antisense oligonucleotides show promise in mouse models of SMA.
This project involves testing a novel non-viral SMN gene therapy approach for SMA using immunogenes. Immunogenes consist of motor neuron targeting antibodies complexed with gene expression plasmids. The therapeutic effects of SMN immunogenes will be evaluated on clinical progression, neuropathology, and SMN splicing and axonal functions in a mouse model of SMA. This project will employ knockout mice, behavioural studies, confocal microscopy, immunohistochemical and biochemical techniques
Development of bifunctional peptide-oligonucleotide conjugates as a novel RNA based therapy for C9orf72 amyotrophic lateral sclerosis
Supervisors: Dr Fazel Shabanpoor, Dr Bradley Turner
Project Site: Florey Institute of Neuroscience and Mental Health
Contact: Dr Fazel Shabanpoor T: 9035 7273 E: fazel.shabanpoor@unimelb.edu.au
Project description: Amyotrophic lateral sclerosis (ALS) is an incurable disease of motor neuron degeneration in the brain and spinal cord, leading to paralysis of voluntary muscles and death by respiratory failure within a median of 3 years from onset(1). The expansion of a GGGGCC (G4C2) hexanucleotide repeat in the first intron/promoter of C9orf72 gene has been reported to be the most common genetic cause of familial and sporadic ALS and frontotemporal dementia (FTD)(2, 3). A gain-of-function as a results of sequestration of RNA-binding protein by toxic RNA resulting from the expanded G4C2 repeat has also been proposed (4). Another causal mechanism is the repeat-associated non-ATG (RAN) translation of the intronic G4C2 repeat expansion in both sense and antisense direction which can generate up to five different dipeptide repeats proteins that can form toxic aggregates (4) (Figure. 1A).
The aim of this project is to use oligonucleotide-based therapeutic approach to selectively degrade C9orf72 sense and antisense RNAs with repeat expansion (Fig. 1B) The sense and antisense oligonucleotides will be conjugated to a single cell-penetrating peptide for their simultaneous intracellular delivery. Using this novel therapeutic strategy, the level of repeat-expanded C9orf72 RNA transcripts will be reduced. This approach will mitigate the main pathological hallmark of ALS, repeat-expanded RNA and aggregated protein toxicities.
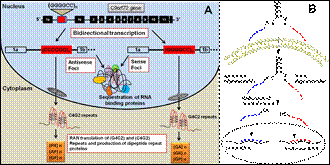
Figure.1: (A) Schematic illustration of bidirectional transcription of chromosome 9 open reading frame (C9orf72) gene. Formation of sense and antisense foci and RAN translation of the G4C2 and C4G2 repeats. (B) Delivery of sense and antisense oligonuclotides conjugated to a single cell-penetrating peptide for simultaneous knockdown of sense and antisense C9orf72 RNAs with repeat expansion.
Skill acquisition: A broad range of skills will be acquired. Students will be trained in synthesis of peptides, conjugation of peptides to oligonuclotides, HPLC purification, mass spectrometry characterization. Cell culture, RNA extraction, RT-qPCR, protein purification and western-blotting and immunohistochemistry.
Development of autophay-inducing peptides as therapy for neurodegenerative diseases
Supervisors: Dr Fazel Shabanpoor, Dr Bradley Turner
Project Site: Florey Institute of Neuroscience and Mental Health
Contact: Dr Fazel Shabanpoor T: 9035 7273 E: fazel.shabanpoor@unimelb.edu.au
Project description: The altered protein degradation and accumulation of misfolded, aggregate-prone proteins is one of the main hallmark of neurodegenerative diseases. Autophagy is an intracellular process which plays a major role in clearance of misfolded/aggregate-prone protein. Motor neurons are in particular very vulnerable to the accumulation of misfolded proteins. Due to their inherent low autophagy capacity, motor neurons can clear their aggregated protein and undergo degeneration (1).
Recent identification of an autophagy-inducing peptide (Fig. 1) has provided a platform for development of autophagy-inducing peptide drugs with potential therapeutic application for neurodegenerative diseases (2). However, these newly discovered peptides have low efficacy and also poor cell-permeability. They are not capable of crossing cell membrane to reach their target in the cytosol (Fig. 1). Therefore, the aim of this project is to (i) develop analogues of beclin 1 peptide with higher autophagy-inducing efficacy and (ii) to enhance their cell uptake by conjugating them to cell-penetrating peptide.
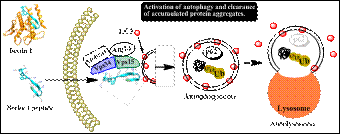
Figure.1: Cellular uptake of Beclin 1 peptide, activation of autophagy and clearance of aggregated proteins.
Skill acquisition: A broad range of skills will be acquired. Students will be trained on synthesis peptides, HPLC purification, mass spectrometry characterization. Cell culture, protein purification, western-blotting, immunofluorescent and immunohostochemistry.
Bioanalytical tools to investigate the role of metalloproteins in Alzheimer’s disease and amyotrophic lateral sclerosis
Supervisors: Dr. Blaine Roberts
Project Site: Florey Inst. Neuroscience-Melbourne Brain Centre
Contact: Dr. Blaine Roberts blaine.roberts@florey.edu.au
Project description: Trace elements are an essential requirement for life. Transition elements, including copper (Cu), iron (Fe) and zinc (Zn), are used to catalyse a wonderful array of reactions throughout all kingdoms of nature. It is then no surprise that the most complex organ to have evolved, the brain, is a rich source of transition metal chemistry. However, we still lack the detailed understanding of how transition elements and the biomolecules that rely on them are involved in the function of the brain. Alzheimer’s disease and amyotrophic lateral sclerosis both have a rich history indicating a critical role of trace elements Cu, Fe, and Zn in their pathophysiology. My lab has implemented bioanalytical tools that allow us to investigate the role metalloproteins have in neurodegeneration. This has project will investigate the role of metalloproteins in the neurodegenerative process.